Planetary was founded on a love for natural and marine environments, and a drive to protect what we see as endangered. While we’re still in the process of learning, we have taken every step we can to ensure that our efforts never threaten the very environments that we are trying to restore.
We acknowledge that introducing any substance to a marine environment can carry risks if not managed carefully. We take all potential risks seriously and have designed our process to minimise them.
This page lists the key concerns about OAE projects that have been identified over decades of research into available materials and methods, and it describes how Planetary addresses those concerns.
We work with third party scientific and academic partners to ensure that our products and methods are safe for both humans and the marine environment, and we implement extensive monitoring and testing throughout our process to identify unexpected changes.
Safe and Established Materials
Our process uses naturally occurring antacids that are non-toxic and that do not accumulate in organisms’ tissues over time.
Our current projects use magnesium hydroxide, an antacid that is well-studied and well-understood. It has been in use for nearly a century, and has been the subject of rigorous safety testing by dozens of independent studies.
Lab and field experiments have clearly established safety thresholds for its use in marine systems.
Planetary applies these studies, as well as the information from material safety data sheets and regulations, to our products and processes.
pH Changes
We use minerals that dissolve slowly, preventing sudden changes in pH that could harm marine life.
Magnesium hydroxide takes days or weeks to fully split into its component parts. This means that the hydroxide – the ion which works to counteract acidic CO2 – is released into the seawater slowly, and only after the magnesium hydroxide has dispersed into the receiving waters.
The net effect of our addition to wastewater is to make it more similar to the seawater it flows into. Since wastewater is made of fresh water and contains biological CO2 after treatment, it’s slightly more acidic than seawater. Adding magnesium hydroxide has the effect of balancing it to make it the same pH as the sea.
Low Doses
All our field projects begin with a very low concentration of antacid, so that the addition does not disturb a marine environment even at the point of release.
As soon as the material reaches the ocean, it disperses very quickly, in many cases becoming about 1,000 times less concentrated within 50-100 metres of the outfall. The tide and currents then wash the plume away from the area, diluting the antacid more.
This means that even a short way away from the addition point, the concentration is more than 700,000 times more dilute than the milk of magnesia you buy in a store.
When our projects scale up, they do so incrementally and only after rigorous, detailed research and meaningful public participation. They never exceed established safety thresholds or permitting limits.
Minimising Physical Disruptions
Our process minimises potential disturbances to marine environments by using permitted ocean outfalls and established infrastructure.
We do not create new ocean outfalls or bring heavy traffic or construction to coastal areas.
No Bioaccumulation
Bioaccumulation occurs when a substance builds up in an organism’s tissues over time and concentrates as it moves up up a food chain, meaning that even very low concentrations of toxic substances can become dangerous.
Magnesium hydroxide does not bioaccumulate.
This question has been investigated directly, partially because magnesium hydroxide is used so broadly in wastewater treatment, and no evidence for MH bioaccumulation has been found. The EU’s European Chemicals Agency declares that magnesium hydroxide “has no bioaccumulation potential.” MH exposure risks do not build over time or up a food chain.
Interaction with Plankton
Phytoplankton do not experience significant impacts from magnesium hydroxide additions.
Recent studies indicate that phytoplankton, the foundation of any ocean food chain, experience very limited impacts when exposed to prolonged increases in alkalinity, even at concentrations more than 100 times higher than Planetary considers for its projects.
Similar studies have been conducted at Dalhousie University in Canada. In scenarios which mimicked the naturally flushed systems where Planetary’s field additions will occur, preliminary results indicate that the phytoplankton species tested were not harmed by the addition of magnesium hydroxide. These studies do note that phytoplankton could be impacted by the addition of any material if it is concentrated enough to block sunlight from reaching phytoplankton.
To ensure we follow the science and because plankton are so important to the ocean ecosystem, Planetary pays particular attention to their health in our ongoing monitoring activities.
When we add alkalinity to water, we monitor both the turbidity (or “cloudiness”) of the water and the plankton communities themselves near the outfall to ensure that there are no unforeseen changes from our addition.
Interaction with Aquatic Animals
Magnesium hydroxide has been shown not to be toxic to aquatic fish and invertebrates.
The gold standard of safety testing for aquatic animals is a test that exposes the substance in question to an acute dose. These tests are completed both with fish and aquatic invertebrates, most commonly with a species of fathead minnows (Pimephales promelas) and a small crustacean known as a water flea (Daphnia magna).
Magnesium hydroxide has been tested against both of these species. Even at concentrations much higher than any of our research projects will consider, the addition was deemed non-toxic. (The European Chemicals Agency has published documentation for MH toxicity tests – see here for fish and here for invertebrates, among other species tests. For detailed results, expand the “reference 1” tables.)
Planetary’s Testing and Monitoring
We utilise rigorous testing protocols to ensure that our projects do not cause unexpected changes in an ecosystem.
This includes:
- Off-site testing for impurities on every batch of alkaline material that we use, rejecting batches that do not pass.
- Sensors deployed throughout the system, around the addition point and in the ocean.
- Surveys of the ocean, sediment, and local biology, conducted before, during, and after each project.
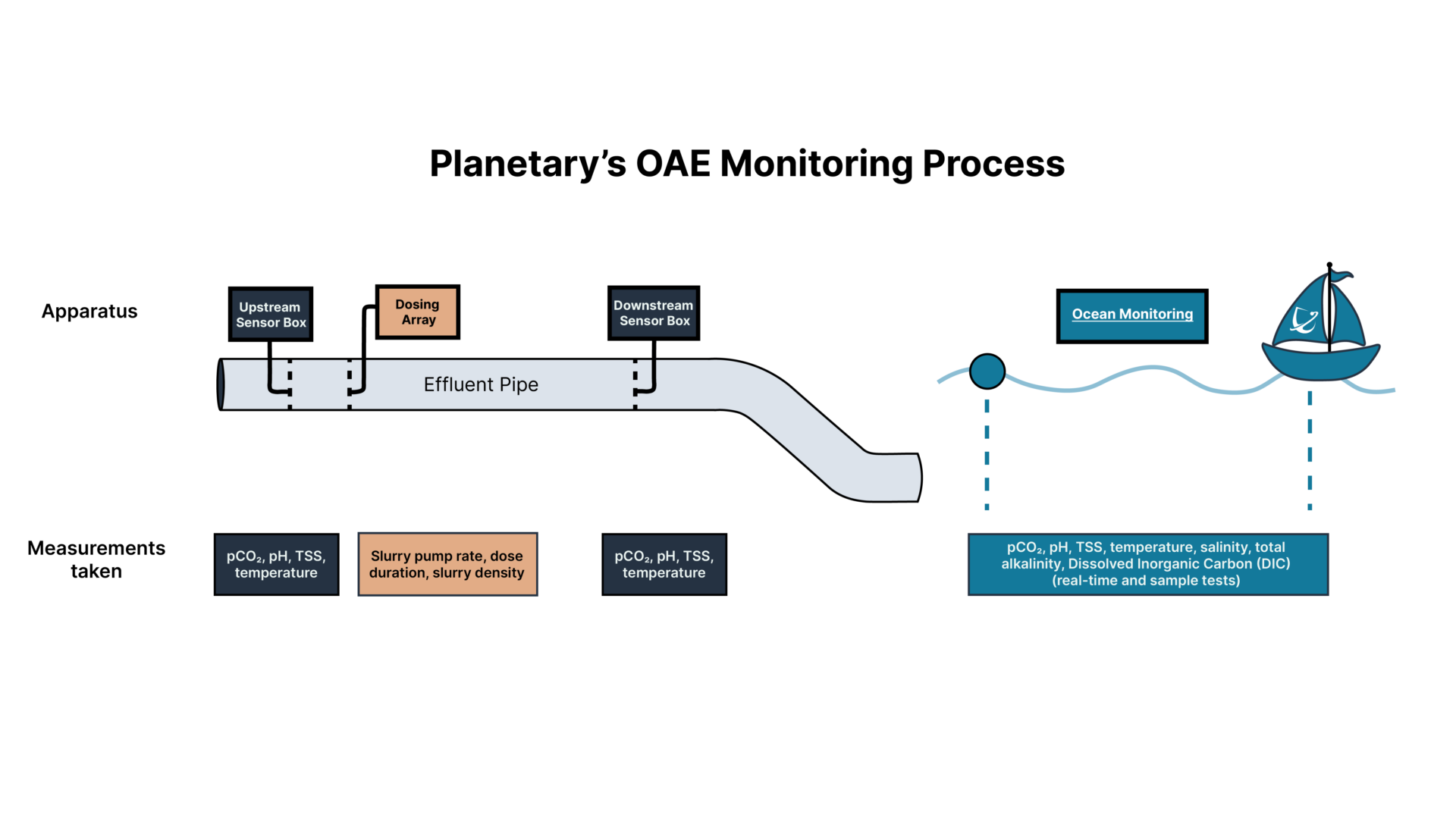
Continuous Monitoring Systems
Sensors deployed upstream and downstream of the addition point and moored in the ocean continually measure the water’s CO2 content and pH level.
We use these measurements to assess carbon removal and acidity, and to validate that we are staying within regulatory safety limits.
This data is sent to on-site operators and the remote Planetary team: if any measure approaches the limit of our permitted activities, we are notified that we may need to reduce dosing rates.
Discrete Sampling Techniques
We regularly collect and analyse water samples from several monitoring points. This enables us to calibrate real-time sensors and to conduct further testing that is not possible on a real-time basis.
These tests include monitoring dissolved carbon and total alkalinity content, as well as monitoring the concentrations of impurities. While the trace metal content of the alkaline material has been tested before the addition, we test here again to identify any unexpected interactions with the effluent so we can be fully confident in the safety of the addition.
We have established additional practices to monitor the biological health of marine systems, and to detect potential ecological disruptions as soon as possible. To learn more about those practices, see our Ocean Monitoring page.
Ocean Monitoring